
Nitrogen compounds have a very long history ammonium chloride having been known to HerodotusThey were well known by the Middle Ages. This ignition can result in an explosion accompanied by.
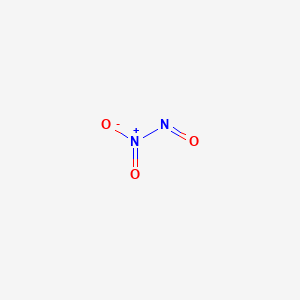
Liquid NITROGEN TETROXIDE is an oxidizing agent consisting of an equilibrium mixture of colorless dinitrogen tetraoxide N2O4 and red-brown nitrogen dioxide NO2.
Dinitrogen tetroxide toxicity. Liquid NITROGEN TETROXIDE is an oxidizing agent consisting of an equilibrium mixture of colorless dinitrogen tetraoxide N2O4 and red-brown nitrogen dioxide NO2. The exact composition of the mixture depends on the temperature with higher temperature favoring conversion to NO2. Vaporizes readily to give NO2 also an oxidizing agent.
Noncombustible but can accelerate the burning of. LeadIIIV oxide also called red lead or minium is the inorganic compound with the formula A bright red or orange solid it is used as pigment in the manufacture of batteries and rustproof primer paintsIt is an example of a mixed valence compound being composed. Nitrogen compounds have a very long history ammonium chloride having been known to HerodotusThey were well known by the Middle Ages.
Alchemists knew nitric acid as aqua fortis strong water as well as other nitrogen compounds such as ammonium salts and nitrate salts. The mixture of nitric and hydrochloric acids was known as aqua regia royal water celebrated for its ability to dissolve. Nitrobenzene is an industrial chemical.
It is an oily yellow liquid with an almond-like odor. It dissolves only slightly in water and will evaporate to air. It is produced in large quantities for use in industry.
Nitrous oxide dinitrogen tetroxide oxygen difluoride and chlorine trifluoride. For oxidizing compressed gases in cylinders a unique hazard is that fire can result from the gas passing through a gauge or piping system that is not free of hydrocarbons even in trace amounts. Spontaneous ignition of the hydrocarbon will occur.
This ignition can result in an explosion accompanied by. Dinitrogen tetroxide Dinitrogen tetroxide is an oxidizer and highly toxic and corrosive gas. Dinitrophenol 24-Dinitrophenol is the most important of dinitrophenols six possible isomers resembling a yellow sand-like crystalline solid.
Formula Type Chemical Name N 2 H 6 M Dinitrogen hexahydride HNO 2 A Nitrous Acid CO M Carbon monoxide HCl A Hydrochloric Acid S 4 Cl 8 M Tetrasulfur octachloride CaOH 2 I Cadmium hydroxide H 2 CrO 4 A Chromic acid MgSO 4 I Magnesium sulfate SO 3 M Sulfur trioxide May 06 2013 The bonding in Hydrogen Chloride is covalent but due to the high electro negativity tendency of. Academiaedu is a platform for academics to share research papers. Substancial - Free ebook download as Text File txt PDF File pdf or read book online for free.
Contains some random words for machine learning natural language processing. We always make sure that writers follow all your instructions precisely. You can choose your academic level.
High school collegeuniversity masters or pHD and we will assign you a writer who can satisfactorily meet your professors expectations.