If you decide to polish your plated object use gentle pressure and mild cleaners to avoid polishing off the copper layer. The technique is simple and can be built in house and the overall cost is estimated to range.

Copper is a soft malleable and ductile metal with very high thermal and electrical conductivity.
Is copper oxide and copper chloride in water toxic. It is also commercially practical to combine copperII oxide with an excess of ammonium chloride at similar temperatures producing copper chloride ammonia and water. Citation needed CuO 2NH 4 Cl CuCl 2 2NH 3 H 2 O. Although copper metal itself cannot be oxidised by hydrochloric acid copper-containing bases such as the hydroxide oxide or copperII carbonate can react to form.
CopperII chloride is light brown when anhydrousIt is green when hydratedIt is a weak oxidizing agentIt reacts with aluminium foil to make hydrogen copperI oxide and aluminium chlorideThis is used in school demonstrations. It releases chlorine and turns into copperI chloride when heated very hot. It reacts with sodium hydroxide to make copperII hydroxide.
A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water. Copper is a chemical element with the symbol Cu from Latin. Cuprum and atomic number 29.
It is a soft malleable and ductile metal with very high thermal and electrical conductivityA freshly exposed surface of pure copper has a pinkish-orange colorCopper is used as a conductor of heat and electricity as a building material and as a constituent of various metal alloys such as sterling. The major soluble salts copperII sulfate copperII chloride are generally more toxic than the less soluble salts copperII hydroxide copper II oxide. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis.
Long-term exposure in rats and mice showed no overt signs of toxicity other than a dose-related reduction in growth. After the reaction adding a little water makes the blue solution of copperII nitrate visible. The depth of discussion depends on the level of the students involved.
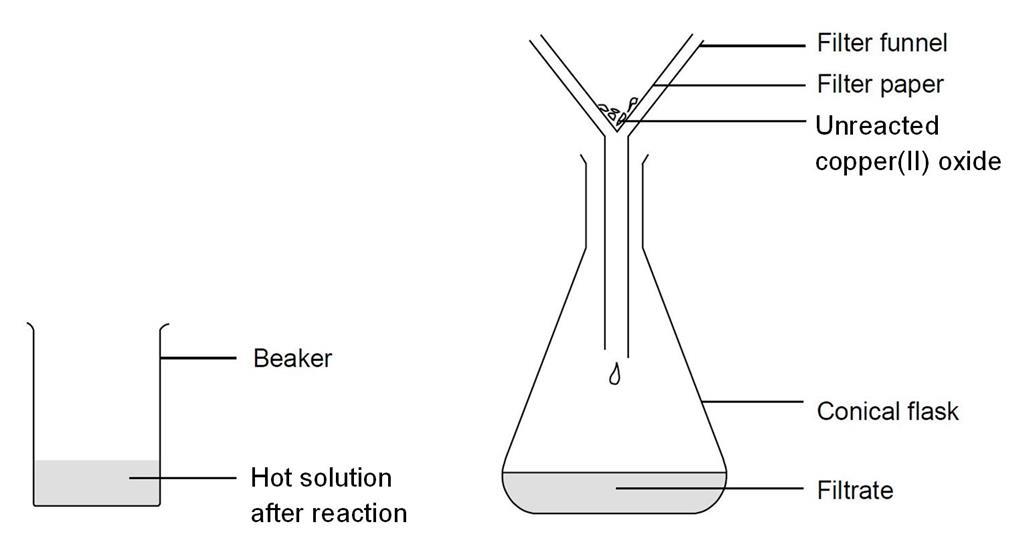
Essentially it is a competition between metal1 and metal2 for oxygen in a reaction represented by. Metal1 Metal2 oxide Metal1 oxide Metal2 The more reactive metal displaces the less reactive. Copper oxide particles are practicably non-soluble at aqueous solutions with a pH 55 and above.
Textile products impregnated with copper oxide particles continue to be efficacious even after 50 repeated home or industrial washings 56 86. The copper oxide particles serve as a reservoir of copper ions. These ions are slowly liberated in the.
Its abundance is 25 x 10-4 mgL in sea water. The Earths copper was formed in exploding white dwarfs and massive stars before the solar system formed. Copper readily forms simple binary compounds which are chemical compounds consisting of only two elements.
Examples of such compounds include copper oxide copper sulfide and copper chloride. The largest users of chlorine are companies that make ethylene dichloride and other chlorinated solvents polyvinyl chloride resins chlorofluorocarbons and propylene oxide. Paper companies use chlorine to bleach paper.
Water and wastewater treatment plants use chlorine to reduce water levels of microrganisms that can spread disease to humans disinfection. This reagent is widely used in the selective flotation of leadcopperzinc and copperzinc ores to depress sphalerite pyrite and certain copper sulfides. Sphalerite rejection from copper concentrates is often of major concern as zinc is a penalty element in copper smelting.
Cyanide chemistry is comprehensively reviewed by Guo et al. Your shiny newly copper plated object will quickly patina as it is exposed to air water salt skin and more. This oxidation can be removed from an unprotected surface with a very light polish.
If you decide to polish your plated object use gentle pressure and mild cleaners to avoid polishing off the copper layer. If you really want to get fancy you can also do reverse electroplating to. Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.
The chemical symbol for Copper is Cu. Copper is a soft malleable and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a reddish-orange color.
Copper is used. Graphene Oxide is a derivative of graphitegraphene invented in 1849 and is well-known to be dangerously toxic to living bodies. Graphene oxide has a direct effect on human neurons and can be controlled from the outside with a magnetic field apparatus.
Graphene Oxide contains semi-conducting properties proportional to the level of oxidation. Its hydrophilic attracted to water and has. ACQ alkaline copper quaternary is a water-based wood preservative that prevents decay from fungi and insects ie it is a fungicide and insecticide.
It also has relatively low risks based on its components of copper oxide and quaternary ammonium compounds. Water-based preservatives like ACQ leave a dry paintable surface. ANHYDROUS HYDROGEN CHLORIDE is an anhydrous no water strong acid.
Reacts rapidly and exothermically with bases of all kinds including amines and amides. Reacts exothermically with carbonates including limestone and building materials containing limestone and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides carbides borides and phosphides to generate toxic.
Ferrous chloride ferric chloride water. Metallic or elemental copper in boiler deposits is dissolved in the hydrochloric acid solution by the following reaction. FeCl 3 Cu CuCl FeCl 2.
Ferric chloride copper cuprous chloride ferrous chloride. Once cuprous chloride is in solution it is immediately redeposited as metallic copper on the steel surface according to the following reaction. Bromine chlorine copper mercury fluorine iodine and silver.
Alkaline and Alkaline Earth Metals such as calcium lithium magnesium sodium potassium powdered aluminum Carbon dioxide carbon tetrachloride and other chlorinated hydrocarbons water Bromine chlorine fluorine and iodine. Do not use CO2 water or dry chemical extinguishers. Use Class D extinguisher eg Met-L-X or dry.
Ethenyl ethanoate is manufactured by passing a mixture of ethanoic acid vapour ethene and oxygen over heated palladiumll and copperll chlorides. Ethenyl ethanoate and an acrylic ester for example methyl 2-methylpropenoate are then co-polymerized to form. BPCs are also used in calendaring and mechanical drawing processes which are used for the fabrication of copper plates Zinc and steel.
The products of Zinc carbonate BPC are known to be non-toxic with high purity which has high water solubility and is more stable when compared to BPC anhydrous. American Water Works Association ANSIAWWA B408-93 Liquid Polyaluminum Chloride Colorado Dec. RTECS-Registry of Toxic Effects of Chemical Substances On-line search Canadian Centre for Occupational Health and Safety.
Mesityl oxide Methyl ethyl ketone Methyl isobutyl ketone Group 9. Saturated Hydrocarbons Butane Cyclohexane. As explosion or toxic fume or other hazard may result.
Mallinckrodt Specialty Chemicals Co. 589 Incompatible Substances COMPOUNDS INCOMPATIBLE WITH. Acetic acid chromic acid nitric acid ethylene glycol perchloric acid peroxides and permanganates Acetone.
Sodium oxide reacts with carbon dioxide to form sodium carbonate. The chemical equation is given below. 2Na 2 O 3CO 2 2Na 2 CO 3.
Sodium oxide reacts with some acids such as hydrochloric acid to form sodium chloride and water. The chemical equation for this reaction is given below. Na2O 2HCl 2NaCl H2O.
Uses of Sodium Oxide. Ferric chloride coagulation followed by filtration through iron-coated sand has been found to efficiently remove As from drinking water. 2001 applied this technique which was found to remove As to a level below 20 μgL from tube well water with a maximum As concentration of 400 μgL.
The technique is simple and can be built in house and the overall cost is estimated to range. The SEMS Search allows you to retrieve Superfund data from the Superfund Enterprise Management System SEMS database in Envirofacts. Specify a facility by using any combination of facility name and geographic location.