In this experiment due to ease of use and consistent results we will use nitric acid as the oxidizing agent. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas.

Concentrated nitric acid is highly corrosive and causes severe burns if spilled onto your skin.
Is nitric acid gas toxic. Almost all commercial quantities of nitric acid are manufactured by the oxidation of ammonia with air to form nitrogen oxides that are absorbed in water to form nitric acid. Because nitric acid has a maximum boiling azeotrope at 69 wt the processes are usually categorized as either weak subazeotropic or direct strong superazeotropic. Typically weak processes make 50-65 wt acid and.
Nitric acid HNO 3 colourless fuming and highly corrosive liquid freezing point 42 C 44 F boiling point 83 C 181 F that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosivesIt is toxic and can cause severe burns. The preparation and use of nitric acid were known to the early alchemists. Nitric Acid is a strong acid with chemical formula HNO 3.
It is also known as the spirit of niter and aqua fortis. In its pure form it is colourless but as it gets older it turns into a yellow cast. This colour appears due to the decomposition of Nitric acid to oxides of nitrogen and water.
It is highly corrosive and toxic. It causes severe skin burn. It reacts with hydroxides metals and.
Nitric oxide gas can be turned to nitrogen dioxide gas easily. Nitrogen dioxide is a toxic gas. This happnes because nitric acid is an oxidizing acid.
But HCl is not a oxidizng acid. So no toxic gas forming when iron react with HCl acid. Can you explain reactions shown by nitric acid by oxidation numbers.
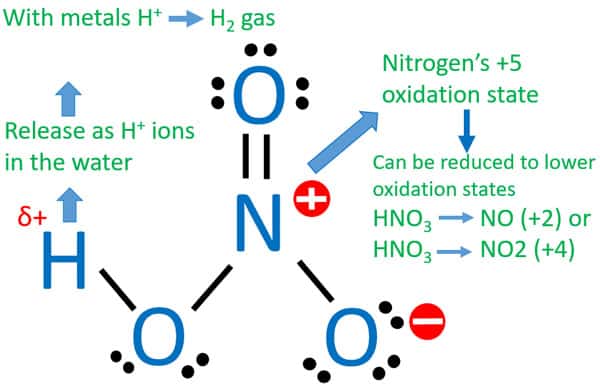
Nitrogen - nitrogen is at 5 oxidation state. It is the maximum oxidation number shown. If the nitric acid is dilute the copper will be oxidized to form copper nitrate with nitric oxide as a byproduct.
If the solution is concentrated the copper will be oxidized to form copper nitrate with nitrogen dioxide as a byproduct. Both nitric oxide and nitrogen dioxide are noxious and potentially toxic at high levels. Nitrogen dioxide is the ugly brown gas present in the smog haze over.
Incompatibilities of concentrated nitric acid. Never mix concentrated nitric acid and organics such as acetone unless you are following a respectable procedure and use a blast shield and proper precautions. Never store mixtures of concentrated acids particularly nitric acid and organic or inorganic waste components.
Immediately dilute any mixtures generated from concentrated acids by slow. Acid rain results when sulfur dioxide SO 2 and nitrogen oxides NO X are emitted into the atmosphere and transported by wind and air currentsThe SO 2 and NO X react with water oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO 2 and NO X that cause acid rain is from. Nitric acid 65 - 70 Revision Date 25-Apr-2019 4. First-aid measures General Advice Immediate medical attention is required.
Show this safety data sheet to the doctor in attendance. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. Immediate medical attention is required.
At the point of exhaust nitric oxide accounts for about 90 of NOx. However it reacts spontaneously with oxygen in the open atmosphere to produce nitrogen dioxide. Nitric oxide is a colourless gas but nitrogen dioxide is an acid pungent smelling brown gas.
Ozone is an unstable gas and is generated as it is required. Aspiration may lead to pulmonary edema. May cause systemic effects.
May cause acute pulmonary edema asphyxia chemical pneumonitis and upper airway obstruction caused by edema. Depending on the conditions the vapor or fumes of nitric acid may actually be a mixture of nitric acid and various oxides of nitrogen. The composition may vary with.
Toxic if inhaled Company Fisher Scientific One Reagent Lane Fair Lawn NJ 07410 Tel. 201 796-7100 Acros Organics One Reagent Lane Fair Lawn NJ 07410 Acute Inhalation Toxicity - Dusts and Mists Category 3 Skin CorrosionIrritation Category 1 A Serious Eye DamageEye Irritation Category 1 Oxidizing liquids Category 3 Corrosive to metals _____ Page 1 8 Category 1 _____ Nitric acid 65 - 70. In atmospheric chemistry NO x is a generic term for the nitrogen oxides that are most relevant for air pollution namely nitric oxide NO and nitrogen dioxide NO 2.
These gases contribute to the formation of smog and acid rain as well as affecting tropospheric ozone. NO x gases are usually produced from the reaction among nitrogen and oxygen during combustion of fuels such as. Nitric oxide appears as a colorless gas.
Noncombustible but accelerates the burning of combustible material. Vapors heavier than air. Very toxic by inhalation and skin absorption.
Heating the containers may cause them to rupture violently and rocket. Nitric oxide is a nitrogen oxide which is a free radical each molecule of which consists of one nitrogen and one oxygen atom. Nitrogen dioxide is a chemical compound with the formula NO 2It is one of several nitrogen oxides.
NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. According to safety data sheets Super 8 is a mixture of 810 sodium hypochlorite in water stronger than household bleach.
Scale Kleen contains 2228 phosphoric acid 1823 nitric acid. About one-fourth of the acidity of rain is accounted for by nitric acid HNO 3. In addition to the natural processes that form small amounts of nitric acid in rainwater high-temperature air combustion such as occurs in car engines and power plants produces large amounts of NO gas.
This gas then forms nitric acid via Equations 4 and 5. Aqua regia is a corrosive acid mixture made by combining nitric acid and hydrochloric acid. The usual ratio of acids is 3 parts hydrochloric acid to 1 part nitric acid.
When mixing the acids it is important to add the nitric acid to the hydrochloric acid and not the other way around. Aqua regia is used to dissolve gold platinum and palladium. Can too much nitric oxide be harmful.
NO is a toxic gas at high concentrations. Inhaled nitric oxide can be dangerous. Sometimes it is used for newborn babies that have respiratory failure due to pulmonary hypertension.
Inhaled nitric oxide side effects can include blurred vision confusion dizziness and sweating. More serious side effects can include rapid heart rate and bluish-colored lips. NOx further oxidises in the atmosphere to form nitric acid HNO 3 vapour which is absorbed directly at ground level where it is converted into nitrate-containing particles or dissolved in cloud droplets.
Deposition can often be at significant distances from the source. It is also one of the precursors for formation of low-level atmospheric ozone which is regionally important as a toxic air. No one would deny the harmful effects of acid rain.
Toxic chemical gases produced by various types of industries volcanos lightning forest fires etc. Are responsible for this sort of acidic rainfall. If the damage continues our future generation will live in the much-polluted earth than what we are living in today.
Read till the end of this article to know what should we do to prevent it. In this experiment due to ease of use and consistent results we will use nitric acid as the oxidizing agent. Concentrated nitric acid is highly corrosive and causes severe burns if spilled onto your skin.
Nitrogen dioxide NO2 fumes are highly toxic and can damage the lungs due to inflammation. Do not breathe NO2 fumes and perform. Our highly accurate gas measurement products are used by a wide variety of industries worldwide.
H₂S Methane CH₄ Nitric oxide NO Nitrogen N₂ Nitrous oxide N₂O Non-methane hydrocarbons NMHC Oxygen O₂ Propane C₃H₆ Sulfur dioxide SO₂ Total Hydrocarbons THC Water vapour Moisture H₂O Hydrocarbon mixtures C₁-C₅ Helium He Nitrogen dioxide NO₂. Oxidizers can also be in the form of gases oxygen ozone liquids nitric acid perchloric acid solutions and solids potassium permanganate sodium chlorite. Some oxidizers such as the organic peroxide family are extremely hazardous because they will burn they are combustible as well as they have the ability to provide oxygen for the fire.
They can have strong reactions which can result. Nitric acid and nitrogen dioxide are reduced to form nitrogen gas and oxygen gas. Catalytic converters also use an oxidative catalyst composed of platinum or palladium.
It helps reduce hydrocarbons HC and carbon monoxide CO.