Potassium Permanganate Benzaldehyde ethylene glycol glycerol sulfuric acid. Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents.

This is our newest publication and has been created to support the school technician profession in Scotland.
Is potassium chlorate toxic. A mixture of potassium chlorate and sodium amide explodes Mellor 8258. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315.
This Potassium Perchlorate contains a small amount of Cabosil to help minimize clumping and keep the mixture free-flowing. Perchlorate is moderately toxic can be absorbed through the skin and breathing protection should be worn when handling fine powder. 8 6 2 in.
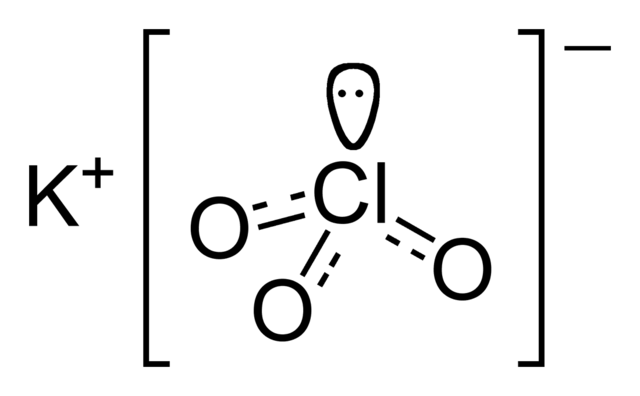
12 reviews for Potassium Perchlorate. Sabath luna verified owner. The chlorate anion has the formula ClO 3.
In this case the chlorine atom is in the 5 oxidation state. Chlorate can also refer to chemical compounds containing this anion. Chlorates are the salts of chloric acid.
Chlorate when followed by a Roman numeral in parentheses eg. Chlorate VII refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair.
Perchlorate ions are somewhat toxic to the thyroid gland. Potassium perchlorate has in the past been used therapeutically to help manage Graves disease. It impedes production of the thyroid hormones that contain iodine.
The perchlorate ion is the least reactive of the generalized chlorates. Perchlorate contains chlorine in its highest oxidation number. A table of.
Sodium chlorate appears as an odorless pale yellow to white crystalline solid. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents.
Contact with wood organic matter ammonium salts sulfur sulfuric acid various metals and. Potassium Iodide MSDS Section 1. Chemical Product and Company Identification Product Name.
Potassium Iodide Catalog Codes. SLP2050 SLP1042 SLP2854 CAS. Potassium Iodide Chemical Formula.
This also applies to potassium chlorate application in matches and fireworks and for potassium nitrate in powder. Potassium alums are bases for paper glue and are applied as a filler of synthetic rubber. Potassium compounds are the most reactive basic chemical compounds which for example applies to potassium hydroxides and nitrates.
Potassium hydroxide forms caustic potash and is applied in. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. This is our newest publication and has been created to support the school technician profession in Scotland.
Potassium Air moisture andor oxygen or water carbon tetrachloride carbon dioxide. Potassium Chlorate Sulfuric and other acids. Potassium Permanganate Benzaldehyde ethylene glycol glycerol sulfuric acid.
Silver and silver salts Acetylene oxalic acid tartaric acid fulminic acid ammonium compounds. Dog Four groups of four female and four male beagle dogs initially 17-21 weeks old were fed diets containing 0 0. Sensitivities vary among individuals.
5 40 400 Nutritional information for Pedialyte 1000 mL and Pedialyte Freezer Pops 62. Aspartame acesulfame potassium Ace-K sucralose neotame advantame and stevia. It is considered as a strong oxidizing agent and does not produce toxic by products.
It is usually prepared from other minerals such as manganese oxide. Structure of Potassium Permanganate KMnO4 The structure of potassium permanganate molecules is illustrated below. Note that this compound features an ionic bond between the potassium cation and the permanganate anion.
Potassium dichromate or anhydrochromate is prepared by adding to the neutral yellow chromate of potassium in solution a moderate quantity of one of the stronger acids. Potassium permanganate is commercially prepared by mixing solution of potassium hydroxide and powdered manganese oxide with oxidizing agents like potassium chlorate. Toxic substances are those which are liable either to cause death or serious injury or to harm human health if swallowed inhaled or by skin contact.
Infectious substances are those which are known or can be reasonably expected to contain pathogens. Dangerous goods regulations define pathogens as microorganisms such as bacteria viruses rickettsiae parasites and fungi or other agents which. The oxidizer is usually ammonium nitrate potassium chlorate or ammonium chlorate and often comprises as much as four-fifths or more of the whole propellant mix.
The fuels used are hydrocarbons such as asphaltic-type compounds or plastics. Because the oxidizer has no significant structural strength the fuel must not only perform well but must also supply the necessary form and rigidity to. All soluble barium compounds are toxic to mammals probably by interfering with the functioning of potassium ion channels.
Barium sulfate BaSO 4 is a white heavy insoluble powder that occurs in nature as the mineral barite. Almost 80 percent of world consumption of barium sulfate is in drilling muds for oil. It is also used as a pigment in paints where it is known as blanc fixe ie.
His matches involved a mixture of potassium chlorate antimony III sulfide gum and starch which ignited when struck on sandpaper. These matches were somewhat unreliable in whether or not they would successfully strike however. In 1830 Charles Sauria a French chemist invented the first phosphorus-based match by replacing the antimony sulfide in Walkers matches with white.
Toxic chlorine vapor is produced. Dont mix chlorine bleach with any acid. Potentially lethal vapors are produced.
The main danger comes from chloramine vapors. Different Brands of One Type of Product. Dont mix different cleaners together.
They may react violently produce toxins or become ineffective. Highly Alkaline Products With Highly Acidic Products. Toxicity criteria on chemicals evaluated by OEHHA.
OEHHA chemical database meta data Export database as CSV file If you are having trouble with the download and would like a copy of the database just drop me LaurieMonserratoehhacagov a note and I will provide you a csv file. Likely to give off flammable or toxic gas when coming in contact with water. This document is for general guidance only and does not create any legally-enforceable rights or obligations.
Aluminum powder Magnesium Lithium Lithium hydride Potassium Sodium Class 5 Oxidizing Substances Organic Peroxides. Oxidizing materials are liquids or solids that readily. There is not normally any need ot make oxygen in the laboratory as it is readily available commercially or through in-house air liquefaction plantsHowever the decomposition of potassium chlorate is one route to O 2 and decomposition of potassium permanganate is another.
In addition electrolysis of KOH using nickel electrodes gives clean oxygen. Appendix A Hazardous Materials Table. Postal Service Mailability Guide.
The mailing information in this table is based on the online DOT Hazardous Materials Table in. Potassium chlorate or fused ammonium nitrate may explode on impact. Apply dry chemical dry sand or special powder extinguishing Class D media.
Do NOT use water carbon dioxide or foam on molten metals. Water may be ineffective for extinguishing a fire but should be used to keep fire-exposed billets ingots and castings cool. If possible move material.
Possible hazardous reactionsReacts explosively with potassium chlorate or bromine trifluoride. Safety Data Sheet according to 29CFR19101200 and GHS Rev. 3 Effective date.
01062015 Page 5 of 7 Ammonium ChlorideLab Grade Created by Global Safety Management Inc. Invented by Jean Chancel in 1805 it consisted of a match head made of a mixture of potassium chlorate sulfur gum arabic and sugar. When the match tip was dipped into a vial of sulfuric acid the match ignited.
However this kind of match was expensive and was also relatively dangerous so Chancels matches never really became widely adopted or in commonplace use. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL.
Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL.