
Evaluate patient carefully to determine the appropriate dose. When changing from parenteral to oral dose forms dose adjustments may be necessary because of pharmacokinetic variations in percentage of digoxin absorbed.
Severe Evidence for interaction.
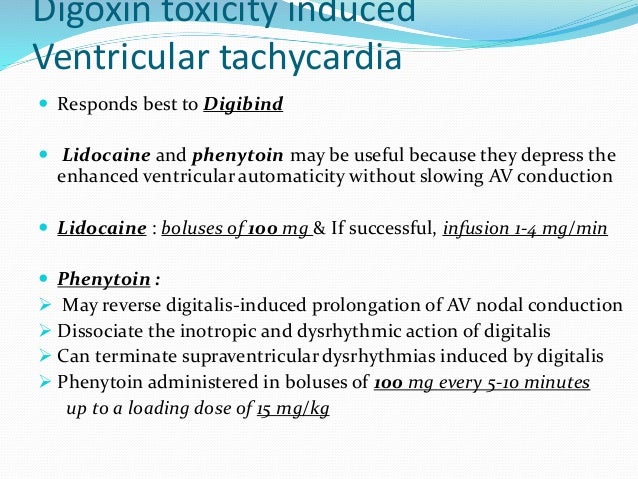
Phenytoin dose in digoxin toxicity. Intravenous formulation is drug of choice for arrhythmias caused by cardiac glycoside toxicity. Second choice drug to carbamazepine. Phenytoin has a narrow therapeutic index.
Its therapeutic range for an anticonvulsant effect is 1020 μgmL and for an antiarrhythmic effect 1020 μgmL. The most common cause of phenytoin. Based on the Beers criteria which strongly recommend against taking more than 0125 mg daily of digoxin in heart failure a dose reduction is recommended.
This patients toxicity management included digoxin antibody fragment. Digoxin antibody fragment use should be considered in the context of life-threatening dysrhythmias a potassium. Phenytoin is predicted to decrease the concentration of digoxin.
Manufacturer makes no recommendation. Ritonavir increases the concentration of digoxin. Manufacturer advises adjust dose and monitor concentration.
Severe Evidence for interaction. Both digoxin and rivastigmine can increase the risk of. When changing from parenteral to oral dose forms dose adjustments may be necessary because of pharmacokinetic variations in percentage of digoxin absorbed.
100 mcg 01 mg digoxin injection 125 mcg 0125 mg tablet or 125 mcg 0125 mg of elixir. Over time the use of digoxin has become less common and as a result the incidence of digoxin toxicity has also been on the decline. This can also be attributed to improved technology in the detection of digoxin levels as well as increased knowledge of various drug interactions.
Nevertheless digoxin use is prevalent enough with a narrow therapeutic window and toxicity continues to be a. Postpone in those with digoxin toxicity. When switching from IV digoxin to oral digoxin and vice-versa the differences in bioavailability between the preparations need to be considered.
Reduce the dosage by about 20 to 25 when switching from oral tablets or solution to IV. Not recommended in patients with acute myocardial infarction heart attack. Has been administered to pregnant.
Drug Name Generic Name. Digitek Lanoxicaps Lanoxin Classification. Cardiac glycoside Cardiotonic Pregnancy Category C Dosage Route Patient response is quite variable.
Evaluate patient carefully to determine the appropriate dose. ADULTS Loading dose 075125 mg PO or 0125025 mg IV. Maintenance dose 0125025 mgday PO.
Lanoxicaps capsules Loading dose 04. Combination of digoxin with any of these drugs would warrant decrease in dose of digoxin to prevent toxicity. Increased risk of cardiac arrhythmias.
Thyroid hormones metoclopramide penicillamine. Decreased therapeutic effects of digoxin. Increasing the dose of digoxin is important.
Cholestyramine charcoal colestipol antacids bleomycin cyclophosphamide. Monitor serum phenytoin levels. Concomitant use may increase digoxin blood levels especially in.
Monitor serum digoxin levels. Concomitant use may potentiate hypoglycemic effects. Monitor blood glucose more frequently.
Monitor for hematologic toxicity. Adverse effects and toxicity. Digitoxin exhibits similar toxic effects to digoxin.
Inducers of CYP3A4 or P-gp such as phenytoin rifampicin and St Johns Wort. Substances that bind digitoxin in the gut such as aluminium containing antacids or colestyramine. Pharmacology Mechanism of action.
Digitoxin inhibits the sodium-potassium ATPase in heart muscle cells resulting in increased force. Digoxin is approximately 70-80 absorbed in the first part of the small bowel. 6 The bioavailability of an oral dose varies from 50-90 however oral gelatinized capsules of digoxin are reported to have a bioavailability of 100.
10 Tmax or the time to reach the maximum concentration of digoxin was measured to be 10 h in one clinical study of healthy patients taking 025 mg of digoxin with. The standard starting dose is 240 mg once a day. For people new to verapamil therapy consider halving the initial dose to 120 mg.
The maximum dose is 240 mg twice a day doses spaced by an interval of 12 hours. Doses for dihydropyridine calcium-channel blockers are shown in the table. Initially 20 to 80 mg PO as a single dose.
May repeat dose in 6 to 8 hours. Titrate upward in 20 to 40 mg increments. The usual dosage is 40 to 120 mgday Max.
If doses exceeding 80 mgday are administered chronically careful clinical and laboratory monitoring are recommended. Heart failure guidelines recommend adding a loop diuretic to standard therapy for reduced ejection.