Empirical Formula Hill Notation. For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine.
C 25 H 42 N 7 O 17 P 3 S xLi CAS No.
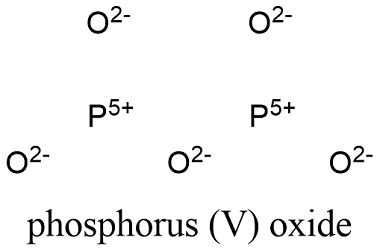
Phosphorus pentoxide formula. Phosphorus pentoxide is a chemical compound with molecular formula P 4 O 10 with its common name derived from its empirical formula P 2 O 5. This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most familiar one a metastable form shown in. Phosphorus pentoxide reacts violently with the following.
Ammonia hydrofluoric acid oxygen difluoride potassium sodium propargyl alcohol calcium oxide sodium hydroxide and chlorine trifluoride. A violent explosion occurs if a solution of perchloric acid in chloroform is poured over phosphorus pentoxide. Phosphorus pentoxide is a chemical compound with molecular formula P 4 O 10 with its common name derived from its empirical formula P 2 O 5.
This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent. Phosphorus pentoxide P 4 O 10 is the acid anhydride of phosphoric acid but several intermediates between the two are known.
This waxy white solid reacts vigorously with water. With metal cations phosphate forms a variety of salts. These solids are polymeric featuring P-O-M linkages.
When the metal cation has a charge of 2 or 3 the salts are generally insoluble hence they exist as. O 10 P 4. Tetraphosphorus decaoxide is a phosphorus oxide.
1 Structures Expand this section. 2 Names and Identifiers Expand this section. Tetra is a prefix that is used for naming four atoms of a formulas nonmetal element.
P 4 O 6 is a molecular formula. Its empirical formula is P 2 O 3. Tetraphosphorus hexoxide is also called phosphorus trioxide.
A binary covalent compound is composed of two different elements usually nonmetals. For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine. Phosphorus particles should glow fluoresce under UV light.
With the exposed areas immersed in cold water to avoid ignition carefully remove all visualized phosphorus particles either loose or imbedded. The use of cold water has the potential to induce hypothermia. Take steps to guard against hypothermia.
Place particles of phosphorus that were removed in cold water-filled containers to. For each of the following questions determine whether the compound is ionic or covalent and write the appropriate formula for it. 11 dinitrogen trioxide N2O3.
14 lithium acetate LiC2H3O2. 15 phosphorus trifluoride PF3. 16 vanadium V oxide V2O5.
17 aluminum hydroxide AlOH3. Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. Phosphorus Pentoxide P4O10 Phophorus Pentoxide P4S3 Phosphorus Sesquisulfide PbC2H3O22 LeadII Acetate PbC2H3O24 LeadIV Acetate PbNO32 LeadII Nitrate PbCl2 LeadII Chloride PbCl4 LeadIV Chloride PbCrO4 LeadII Chromate PbI2 LeadII Iodide PbO LeadII Oxide PbO2 LeadIV Oxide PBr3 Phosphorus Tribromide PbS LeadII Sulfide PCl3. Empirical Formula Hill Notation.
C 25 H 42 N 7 O 17 P 3 S xLi CAS No. 83762 free acid basis Compare Product No. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Melting Point C Physical Form. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More.