
Although DEA was the most active catalyst with the lowest reactions time but the amine content was increased. 22 In many cases the energy difference may be close to zero.
Piperonyl butoxide was used to inhibit cytochrome p-450 and buthionine sulfoximine to inhibit glutathione synthesis.
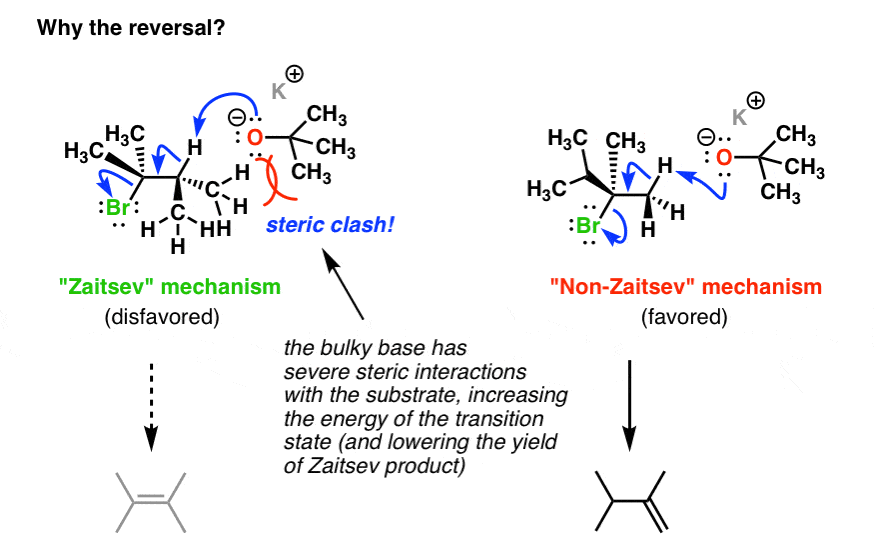
Secondary carbon selectivity t butoxide. Potassium tert-Butoxide KOt-Bu Is A Bulky Base Todays reagent potassium tert-butoxide KOt-Bu is a strong base just like all alkoxides but theres something about it that makes it special. If youve come across the tert-butyl group before you should be able to remember one main thing. Its really darn fat although in these more sensitive times bulky is the preferred.
Carbon dioxide Carbon monoxide. Carbon Secondary hydrogen Secondary structure. Tert-butoxide Tert-butyl carbocation Tert-butyl group Tertiary Tertiary alcohol Tertiary alkyl halide Tertiary amide Tertiary amine Tertiary ammonium salt Tertiary carbocation Tertiary carbon Tertiary.
Look at the carbon that contains the best leaving group. Typically this is Cl Br I or some other group that can act as a good leaving group. Is this carbon primary secondary or tertiary.
Given what we know about SN1 SN2 E1 and E2 reactions we can say the following. The big barrier to the SN2 reaction is steric hindrance. Although carbamates display close similarity to amides they show preference for the anti-isomer conformation.
28 The anti rotamer is usually favored by 1015 kcal mol 1 for steric and electrostatic reasons with respect to the syn counterpart. 22 In many cases the energy difference may be close to zero. As a result those carbamates are found as an approximately 5050 mixture of syn.
Aldol condensations are important in organic synthesis because they provide a good way to form carboncarbon bonds. For example the Robinson annulation reaction sequence features an aldol condensation. The WielandMiescher ketone product is an important starting material for many organic syntheses.
Aldol condensations are also commonly discussed in university level organic chemistry. David Rawn in Organic Chemistry Study Guide 2015 166 The Williamson Ether synthesis. The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether.
The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing elimination. Academiaedu is a platform for academics to share research papers. The use of a tertiary amine as base was crucial for the selective formation of the boron-carbon bond.
The reaction tolerates a variety of functional groups such as carbonyl cyano and nitro groups. Chem 2000 65 164-168. A rhodium-catalyzed borylation with excellent selectivity for C-I bonds provides aryl boronate esters under mild.
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes olefins by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their.
Predict the product of the following reaction. We present an introduction to MS-PCET and a practitioners guide to reaction design with an emphasis on the unique energetic and selectivity features that are characteristic of this reaction class. We then present chapters on oxidative NH OH SH and CH bond homolysis methods for the generation of the corresponding neutral radical species.
Then chapters for reductive PCET. With methanol ethanol isopropanol t-butanol 2 32 and t-amyl alcohol diene 4 was produced in 33 58 62 75 and 68 yields respectively with the over-reduced product 5 in 4 to 11 yields entries 2 to 6. To study the importance of the acidity of the alcohol we tested 222-trifluoroethanol and 111333-hexafluoroisopropanol which afforded yields of 52 and 26 respectively.
Predict the product of the following reaction. Compound 43 had relatively rapid clearance in human liver microsomes with T 12 05 h. Compared with 43 41 and 45 were more stable.
The metabolic stability of compound 9 was comparable to those of 8 10 and 36 but it had low solubility of 79 μgmL. And compound 7 was more stable than the other compounds T 12 120 min. TiOBu 4 showed high selectivity to glycolysis with low primary amine content but its stability can be affected by humidity oxygen or temperature decreasing its activity.
Although DEA was the most active catalyst with the lowest reactions time but the amine content was increased. Complete degradation at low reaction time the lowest primary amine content high selectivity and high. Piperonyl butoxide was used to inhibit cytochrome p-450 and buthionine sulfoximine to inhibit glutathione synthesis.
13-dichloropropene 300 mgkg administered with a stomach tube significantly increased plasma glutamate oxaloacetate transaminase and glutamate pyruvate transaminase activities as well as hepatic centrilobular swelling 15 hr after administration. No significant changes were.