The document includes a policy statement to waive all acute lethality dermal studies for formulated pesticide products see section 30. A sample injection volume of 5 uL is suggested.

The document includes a policy statement to waive all acute lethality dermal studies for formulated pesticide products see section 30.
Test for hazard potential acute toxicity. Acute Dermal Toxicity This method provides information on health hazard likely to arise from short-term exposure to a test chemical by dermal route. Test chemicals should not be administered at doses that are known to cause marked pain and distress due to potential corrosive or severely irritant actions. Glyphosate is a phosphonic acid resulting from the formal oxidative coupling of the methyl group of methylphosphonic acid with the amino group of glycineIt is one of the most commonly used herbicdes worldwide and the only one to target the enzyme 5-enolpyruvyl-3-shikimate phosphate synthase EPSPSIt has a role as an agrochemical an EC 25119 3-phosphoshikimate 1.
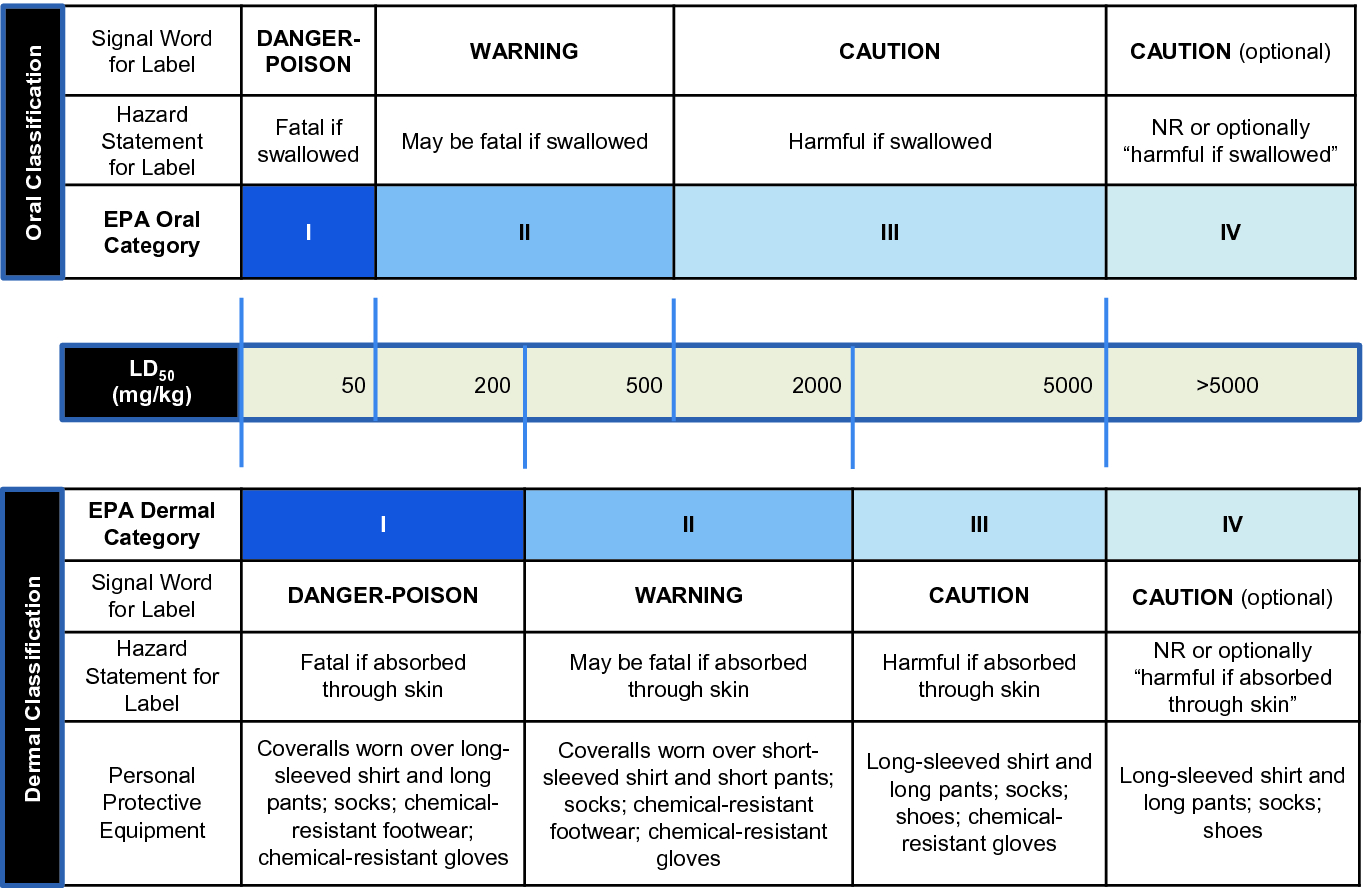
- where expert judgement confirms reliable information indicating the potential for significant acute effects from other animal studies. Recognising the need to protect animal welfare testing in animals in Category 5 ranges is discouraged and should only be considered when there is a strong likelihood that results of such a test would have a direct relevance for protecting human health. The toxicity of a particular pesticide is determined by subjecting test animals to varying dosages of the active ingredient ai and each of its formulated products.
The active ingredient is the chemical component in the pesticide product that controls the pest. By understanding the difference in toxicity levels of pesticides a user can minimize the potential hazard by selecting the. Guidance for Waiving Acute Dermal Toxicity Tests for Pesticide Formulations and Supporting Retrospective Analysis published in 2016 expands the potential for data waivers for acute dermal studies for formulated products.
The document includes a policy statement to waive all acute lethality dermal studies for formulated pesticide products see section 30. A gas chromatographic method for the analysis of N-propyl acetate consists of a stainless steel column 3m x 3 mm packed with chromosorb WHP 100120 mesh coated with 5 FFAP with hydrogen-air flame ioniztion delection and nitrogen as the carrier gas at a flow rate of 30 mLmin is a NIOSH approved method. A sample injection volume of 5 uL is suggested.
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism such as an animal bacterium or plant as well as the effect on a substructure of the organism such as a cell cytotoxicity or an organ such as the liver hepatotoxicityBy extension the word may be metaphorically used to. Glyphosate is an aminophosphonic analogue of the natural amino acid glycine and like all amino acids exists in different ionic states depending on pHBoth the phosphonic acid and carboxylic acid moieties can be ionised and the amine group can be protonated and the substance exists as a series of zwitterionsGlyphosate is soluble in water to 12 gL at room temperature.
By acute toxicity testing. This TG is intended to investigate effects on a very broad variety of potential targets of toxicity. It provides information on the possible health hazards likely to arise from repeated exposure over a relatively limited period of time including effects on the nervous immune and endocrine systems.
Regarding these particular endpoints it should identify chemicals.