
Assess need for administering TIG for prophylaxis. Rarely have cases of tetanus occurred in persons with a documented primary series of tetanus toxoid.
The MVX code reflects the manufacturer of the specific instance of the vaccine.
Tetanus antitoxin administration. Tetanus and Diphtheria Vaccine Dosage and Administration. For intramuscular use only. Diphtheria and Tetanus Toxoids Adsorbed is approved for administration as a 5 dose series at 2 4 6 15-18 months and 4-6 years.
The first dose of Diphtheria and Tetanus Toxoids Adsorbed may be administered as early as 6 weeks of age. Vaccinations or inoculations are excluded as immunizations unless they are directly related to the treatment of an injury or direct exposure to a disease or condition such as anti-rabies treatment tetanus antitoxin or booster vaccine botulin antitoxin antivenin sera or immune globulin. When billing the tetanus vaccine for treatment of an injury or direct exposure to a.
Of 20 adults with less than 00025 unitsmL of tetanus antitoxin in pre- immunization serum 14 70 had antitoxin concentrations of 001 or greater after 2 doses of TDVAX 2 Lf tetanus toxoid dose. It can be inferred from protective antitoxin levels that a complete tetanus toxoid series has an efficacy of almost 100. In the series of 233 cases from 20012008 only 7 cases 3 had received a complete tetanus toxoid series with the last dose within the last 10 years.
Antitoxin levels decrease with time. By 10 years after the last dose most persons have antitoxin levels that only. Co-administration with other vaccines.
Do not mix DTPa- or dTpa-containing vaccines or dT vaccine with any other vaccine in the same syringe unless specifically registered for use in this way. Tetanus-containing vaccines can be co-administered with other vaccines at the same schedule point using separate injection sites. Tetanus-containing vaccines can be co-administered with most other.
Once inside the neurons tetanus toxin cannot be neutralised by antitoxin. The tetanus toxin acts on four areas of the nervous system. A the motor end plates in the skeletal system.
B the spinal cord. And d the sympathetic system. The toxin blocks the release of the inhibitory neurotransmitters glycine and gamma-amino-butyric acid in the central nervous system.
Rarely have cases of tetanus occurred in persons with a documented primary series of tetanus toxoid. Assess need for administering TIG for prophylaxis. TIG provides temporary immunity by directly providing antitoxin.
TIG can help remove unbound tetanus toxin but cannot neutralize toxin that is already bound to nerve endings. Persons who have. Tetanus vaccine also known as tetanus toxoid TT is a toxoid vaccine used to prevent tetanus.
During childhood five doses are recommended with a sixth given during adolescence. After three doses almost everyone is initially immune but additional doses every ten years are recommended to maintain immunity. A booster shot should be given within 48 hours of an injury to people whose.
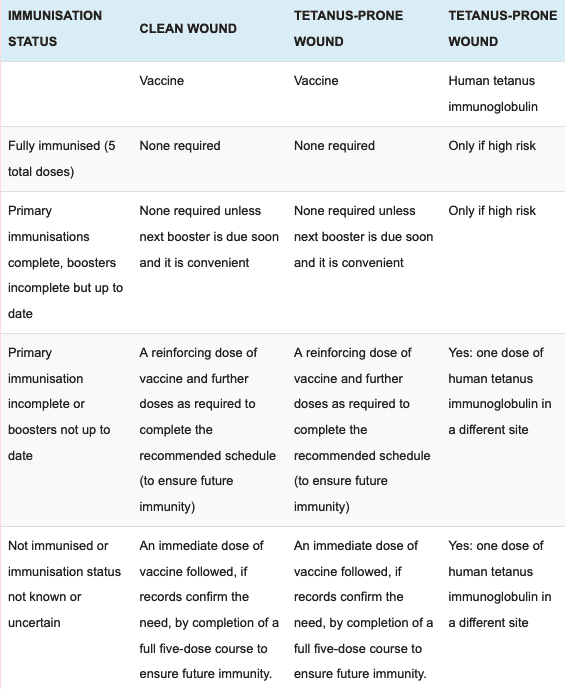
The need for tetanus-containing vaccine in people with a tetanus-prone wound with or without tetanus immunoglobulin depends on the nature of the wound and the persons vaccination history. The doctor may advise you to have a tetanus booster shot depending on how long it is since your last tetanus dose. If you have not had any previous.
Diphtheria is an infection caused by the bacterium Corynebacterium diphtheriae. Most infections are asymptomatic or have a mild clinical course but in some outbreaks more than 10 of those diagnosed with the disease may die. Signs and symptoms may vary from mild to severe and usually start two to five days after exposure.
Symptoms often come on fairly gradually beginning with a sore throat. People who have a tetanus-prone injury and have experienced a severe injection site reaction following a tetanus toxoid-containing vaccine usually have very high serum antitoxin levels and should not receive routine or emergency booster doses of tetanus toxoid-containing vaccine for 10 years following the last dose. A tetanus-prone injury can be defined as an injury significantly contaminated.
Although deaths related to tetanus are exceedingly rare in the Western world where vaccination is common it is estimated that tetanus kills over 1 million people worldwide every year. Once symptomatic wound cleaning and administration of antitoxin where available along with benzodiazepines to control spasms can stabilize the disease. A ventilator may become necessary if breathing.
Administration of human antitetanus immunoglobulin HTIg or equine antitetanus serum is an established practice in the treatment of tetanus. Since the damage caused by tetanospasmin that has entered the nervous system is irreversible much emphasis is placed on neutralizing the circulating toxin before it enters the nervous system. Many authors have explored whether administering these.
Intrathecal administration of antitoxin eg. Via lumbar puncture could inactivate tetanus toxin during its trans-synaptic transport. A meta-analysis indicated that intrathecal administration was superior to the intramuscular route with respect to survival.
Because immunity may not develop after an episode of tetanus vaccination is included in the treatment. -This drug should not replace the usual procedure of debridement tetanus antitoxin penicillin tracheotomy attention to fluid balance and supportive care. If used this drug should be added to the regimen as soon as possible.
To control the neuromuscular manifestations of tetanus. Usual Pediatric Dose for Tetanus. 15 mgkg or 500 mgm2 IV.
Repeat initial dose every 6. Standard for the Administration of Immunizations. Care After Immunization AdolescentAdult 104493 Care After Immunization InfantChild 104495 Biological Product Information.
Contents of Immunizing Agents Available for use in Canada. Botulism Antitoxin Baby BIg Botulism Immune Globulin Intravenous Human Baby BIg Biological Page. Although REVAXIS has not been studied in subjects with tetanus-prone injuries studies have shown that it induces similar tetanus antitoxin titres to Td vaccine.
REVAXIS may therefore be used in subjects with tetanus-prone injuries if concomitant vaccination against diphtheria and poliomyelitis is desirable. The administration of tetanus and diphtheria Tdap boosters given at ages 45 and 65 years is also funded. TIG is issued in ampoules each containing 250 IU of human tetanus antitoxin.
Ampoules of 2000 IU are used for treatment and not for prophylaxis These should be protected from light and stored in a refrigerator at 2C to 8C. They must never be frozen. TIG is given.
Dosage and Administration 22 23 092020 —–INDICATIONS AND USAGE —– BOOSTRIX is a vaccine indicated for active booster immunization against tetanus diphtheria and pertussis in individuals aged 10 years and older. 1 —–DOSAGE AND ADMINISTRATION—– For intramuscular use only. Each dose of BOOSTRIX is administered as a 05-mL injection.
2 An initial dose of. Tetanus antitoxin if dam was not vaccinated during gestation. 30 days after lambing or kidding.
Booster at 45 days 2 weeks later. Clostridium perfringens types C and D antitoxin. Ovine ecthyma for soremouth.
Chlamydia psittaci ewe vaccine. Clostridial 8-way once Caseous lymphadenitis CL. The antibody-containing blood-derived substance was called diphtheria antitoxin and public boards of health and commercial enterprises began producing and distributing it from 1895 onward.
Kitasato von Behring and other scientists then devoted their attention to treatment of tetanus smallpox and bubonic plague with antibody-containing blood products. The use of antibodies to treat. The route of administration.
MVX Codes - Manufacturers of Vaccines. The MVX is an alphabetic string which represents the manufacturer of a vaccine. When MVX code is paired with a CVX vaccine administered code the specific trade named vaccine may be indicated.
The MVX code reflects the manufacturer of the specific instance of the vaccine. For example Pfizer MVX PFR acquired. Licensed biological products with supporting documents is arranged alphabetically by the proper name of the products.
Information is provided for. Reactions to tetanus antitoxin derived from horse serum were especially common but are now rare owing to refinement of the antigenic components. Serum sickness syndrome a serum sicknesslike hypersensitivity reaction occurring after the administration of certain drugs.
It is marked clinically by low-grade fever urticaria facial edema pain and swelling of the joints and lymphadenopathy.