It was initially used in hard contact lens solutions but is now ubiquitous. These studies produced no evidence of an unusual degree or kind of toxicity.
OTHER TOXICITY INFORMATION The relative toxicity of Aliquat 203 didecyl-dimethyl-ammonium-chloride was experimentally investigated in mice rats guinea-pigs rabbits and humans.
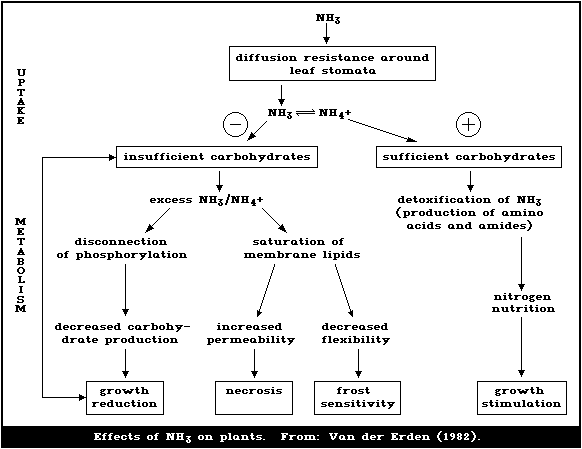
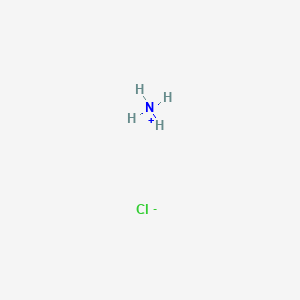
Toxicity of ammonium chloride. The toxicity of ammonium chloride depends on the ammonia which enters the living organism and hence the cell. This substance is readily absorbed by the gastrointestinal tract and utilized in the liver to form amino acids and proteins. When ammonium ions are converted to urea liberated hydrogen ion reacts with bicarbonate ion to form water and carbon dioxide.
The chloride ion displaces the. OTHER TOXICITY INFORMATION The relative toxicity of Aliquat 203 didecyl-dimethyl-ammonium-chloride was experimentally investigated in mice rats guinea-pigs rabbits and humans. These studies produced no evidence of an unusual degree or kind of toxicity.
No data were found which indicated potential toxicologic hazard from wearing of uniforms impregnated with 05 percent Aliquat 203. Acute toxicity inhalation. Ammonium Chloride 12125-02-9 LD50 oral rat 1410 mgkg body weight Equivalent or similar to OECD 401 Rat Male female Experimental value Oral 14 days LD50 dermal rat 2000 mgkg body weight EU Method B3.
Acute toxicity dermal 24 h. Ammonium Chloride Solution is recommended for the lysis of red blood cells RBCs in preparations of human and mouse peripheral blood spleen or bone marrow cells. It is buffered and optimized for gentle lysis of erythrocytes with minimal effect on leukocytes.
It does not contain a fixative agent therefore leukocytes are viable following RBC lysis. Contains 08 NH4Cl 01 mM EDTA in. Benzalkonium chloride BAK otherwise known as alkyl dimethyl benzyl ammonium chloride is a toxic chemical used in cosmetics.
It is a antimicrobial agent deodorant surfactant and a preservative. Benzalkonium chloride has repeatedly demonstrated its toxic characteristics in laboratory experimental and clinical studies. This compound is known to cause extensive damage to the eyes.
Tetramethylammonium chloride is one of the simplest quaternary ammonium salts with four methyl groups tetrahedrally attached to the central N. The chemical formula CH 3 4 N Cl is often abbreviated further as Me 4 N Cl It is a hygroscopic colourless solid that is soluble in water and polar organic solvents. Tetramethylammonium chloride is a major industrial chemical being used.
12125-02-9 Ammonium Chloride ACGIH TLV. 10mgm3 12125-02-9 Ammonium Chloride TWA 10 mgm3 USA. NIOSH 12125-02-9 Ammonium Chloride TWA 10 mgm3 USA.
Safety Data Sheet according to 29CFR19101200 and GHS Rev. 3 Effective date. 01062015 Page 4 of 7 Ammonium ChlorideLab Grade Created by Global Safety Management Inc.
Cetrimonium bromide C 16 H 33NCH 3 3Br. CTAB is a quaternary ammonium surfactant. It is one of the components of the topical antiseptic cetrimide.
The cetrimonium hexadecyltrimethylammonium cation is an effective antiseptic agent against bacteria and fungi. In Meylers Side Effects of Drugs Sixteenth Edition 2016. Quaternary ammonium compounds are surface-active agents.
Some of them precipitate or denature proteins and destroy micro-organisms. The most important disinfectants in this group are cationic surface-active agents such as benzalkonium chloride benzethonium chloride and methylbenzethonium chloride and. The sources of toxicity information for various preservatives are different for example the PCPC in the United States which publishes safety reports known as the Cosmetic Ingredient Review CIR on the basis of independent scientific groups.
Thus Cosmetics EuropeThe Personal Care Association is a similar professional association in Europe. Typically contact dermatitis CD is an. Benzalkonium chloride is a quaternary ammonium compound and it is the most commonly used antimicrobial preservative.
It was initially used in hard contact lens solutions but is now ubiquitous. Currently it can be found in most topical multi-use ophthalmic preparations. BAK is a detergent that denatures proteins and causes lysis of cytoplasmic membranes and is therefore a potent broad.
Chloride the major extracellular anion closely follows the metabolism of sodium and changes in the acid-base of the body are reflected by changes in the chloride concentration. Normally about 80 to 90 of the potassium intake is excreted in the urine the remainder in the stools and to a small extent in the perspiration. The kidney does not conserve potassium well so that during fasting.