For example N 2 O 4 is referred to as dinitrogen tetroxide not dinitrogen tetraoxide and CO is called carbon monoxide not carbon monooxide. Alkanes contain only carbon-carbon single bonds and are the simplest of the hydrocarbons.
6 K 3N potassium nitride.
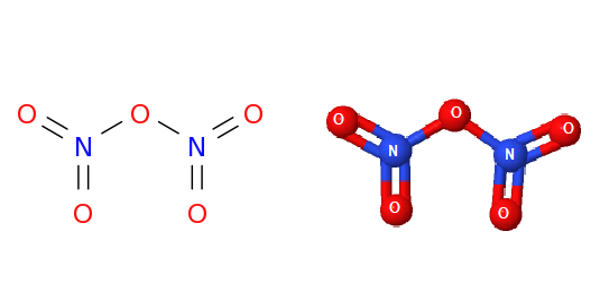
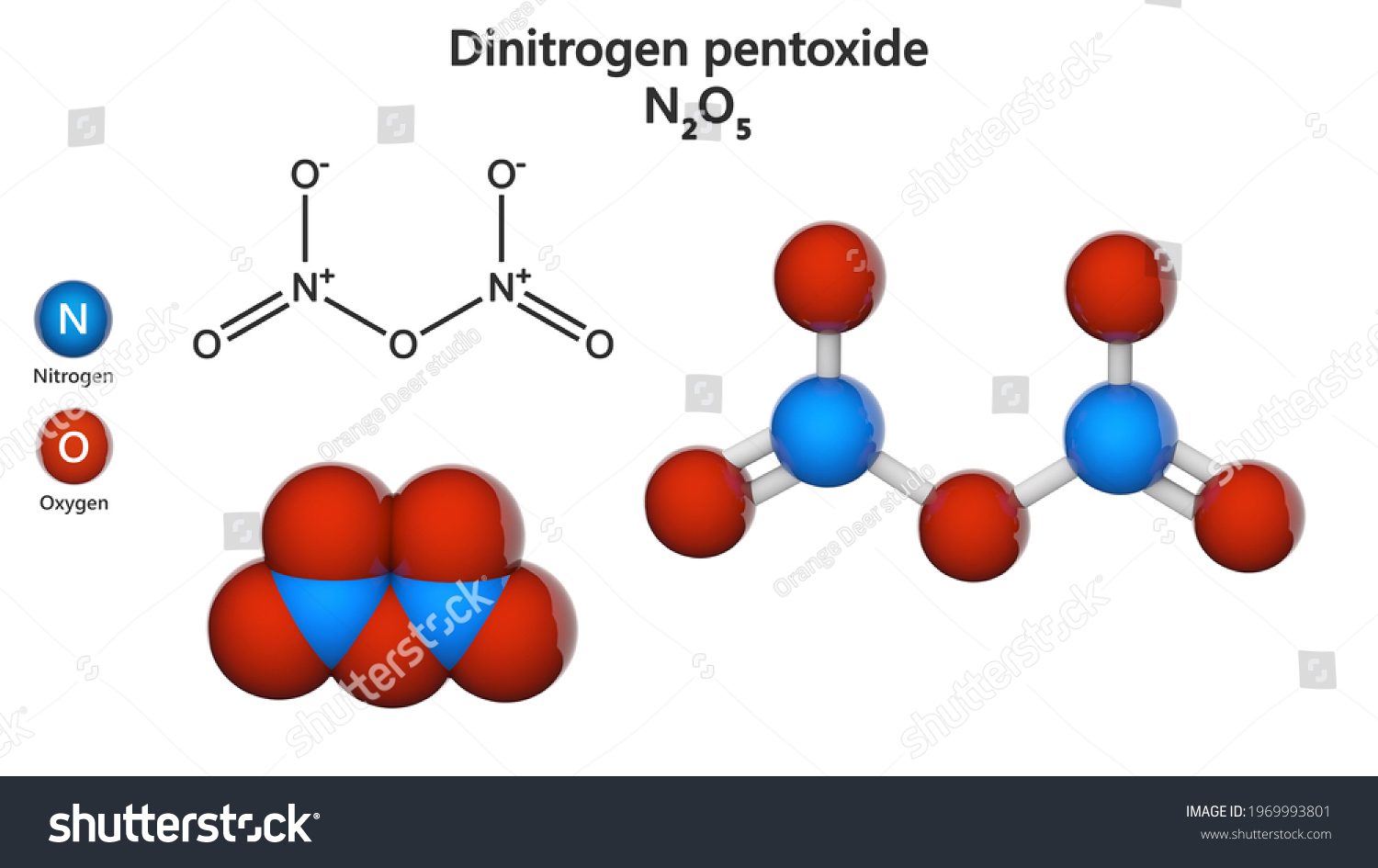
What is dinitrogen pentoxide. Dinitrogen pentoxide is the chemical compound with the formula N 2 O 5 also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 C.
Its boiling point is 47 C and sublimes slightly above room temperature yielding a colorless gas. Dinitrogen pentaoxide is a nitrogen oxide. 1 Structures Expand this section.
2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this section. 4 Related Records Expand.
Dinitrogen tetroxide commonly referred to as nitrogen tetroxide NTO and occasionally usually among ex-USSRRussia rocket engineers as amyl is the chemical compound N 2 O 4It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxideIts molar mass is 92011 gmol. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts.
N 2O dinitrogen monoxide. NO nitrogen monoxide. N2O3 dinitrogen trioxide.
NO2 nitrogen dioxide. 19 N2O4 2 dinitrogen tetroxide. N2O5 dinitrogen pentoxide 11.
PCl3 phosphorous trichloride. PCl5 phosphorous pentachloride. CCl4 carbon tetrachloride.
SiO2 silicon dioxide. 4 TiSO 4 2 titaniumIV sulfate. 5 FePO 4 ironIII phosphate.
6 K 3N potassium nitride. 7 SO 2 sulfur dioxide. 8 CuOH copperI hydroxide.
9 ZnNO 2 2 zinc nitrite. 10 V 2S 3 vanadiumIII sulfide. Write the formulas for the following chemical compounds.
11 silicon dioxide SiO 2. 12 nickel III sulfide Ni 2S 3. 13 manganese II phosphate Mn 3PO 4 2.
Dinitrogen has a the prefix di- which means 2. Therefore there are 2 atoms of nitrogen present. Write dinitrogen as N 2.
Write the chemical symbol for the second element. The second element is the last name of the compound and will follow the first element. For covalent compounds the element name will have a suffix of -ide instead of the normal ending of the.
The first element is named first using the elements name. Second element is named as an Anion suffix -ide 3. Prefixes are used to denote the number of atoms.
To avoid awkward pronunciations the final o or a of the prefix is often dropped when the element name begins with a vowel. For example N 2 O 4 is referred to as dinitrogen tetroxide not dinitrogen tetraoxide and CO is called carbon monoxide not carbon monooxide. Prefixes used in chemical nomenclature prefix number of atoms mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa.
Add an ide to the end of the second compounds name. For both molecular and ionic compounds change the name of the second compound so it ends in ide. Fluorine fluoride.
Dinitrogen tetrahydride reacts with oxygen to produce nitrogen and water. LeadII nitrate reacts with sodium iodide to create lead II iodide and sodium nitrate. Phosphorous reacts with oxygen gas to produce diphosphorous pentoxide.
When calcium comes in contact with water calcium hydroxide and hydrogen gas is produced. When hexane C 6 H 24 reacts with oxygen a combustion reaction occurs. N 2 O 5.
Hydrocarbons contain only carbon and hydrogen and are the simplest type of organic compound a compound containing carbon. Alkanes contain only carbon-carbon single bonds and are the simplest of the hydrocarbons. The simplest of the alkanes are the straight-chain alkanes in which all of the carbon atoms are linked together.
Dinitrogen trioxide phosphorus triiodide phosphorus trichloride arsenic pentabromide iodine trichloride carbon monoxide diphosphorus pentoxide boron trifluoride dichlorine heptoxide carbon tetrabromide nitrogen trihydride pa S TCI 3 Co Cia 07 CßrH. 五酸化二窒素ごさんかにちっそdinitrogen pentoxideとは化学式が N 2 O 5 と表される窒素酸化物である 硝酸の酸無水物に当たり無水硝酸むすいしょうさんとも呼ばれる 窒素の酸化状態.